
i-Fix Dental Implants have been Clinically tested and proven to have excellent osseointegration.









The i-Fix implant system components are made of the biocompatible medical grade, Grade V ELI/ Grade 23 Titanium alloy. The specific Titanium alloy used by
Kamal Medtech was chosen due to its superior properties including high strength to weight ratio, very high corrosion resistance, high modulus of elasticity and its excellent osseointegradtion capabilities.
| Properties | Advantages | |
|---|---|---|
| Tensile Strength | 860 Mpa Minimum | Extremely high strength/ weight ration |
| Elongation | 10% | Improved ductility and fracture toughness under static and dynamic loads |
| Oxygen Content | 0.13% Maximum | |
| Biocompatibility | Excellent | Direct structural and functional connection between bone and implant suface |

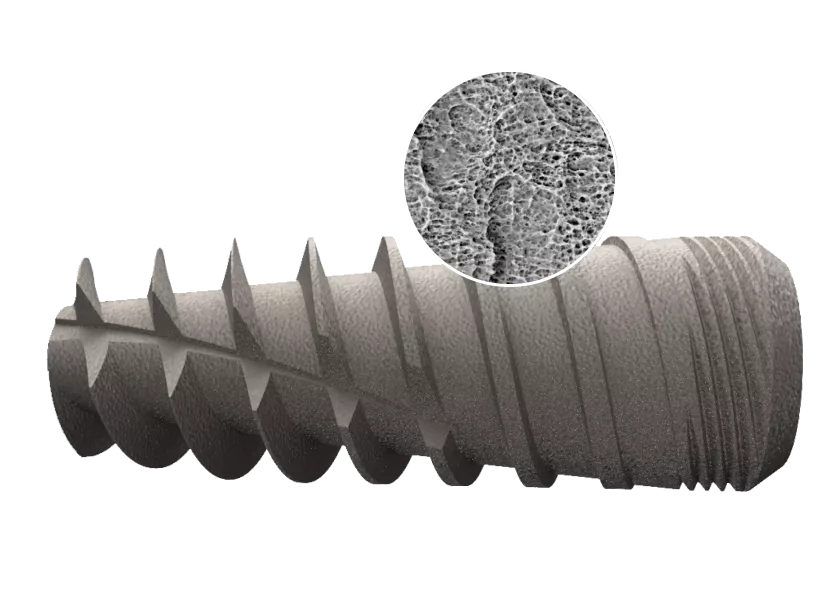
The proprietary i-Fix surface is created by sandblasting, aimed at creating a porous surface topography, followed by triple stage acid etching, intended to generate macro & micro-roughened surface structure. The surface treatment is completed by removing contaminants using ultra-pure water (UPW), a unique process acquired from the semiconductor industry.
Ultramodern manufacturing facility and the quality management system compliant with fifth schedule of MDR 2017 and ISO 13485 Standard.





The term i-Fix C1 refers to a specific type of dental implant system designed for immediate loading of the implant.
Bio-materials are complements for bone augmentation